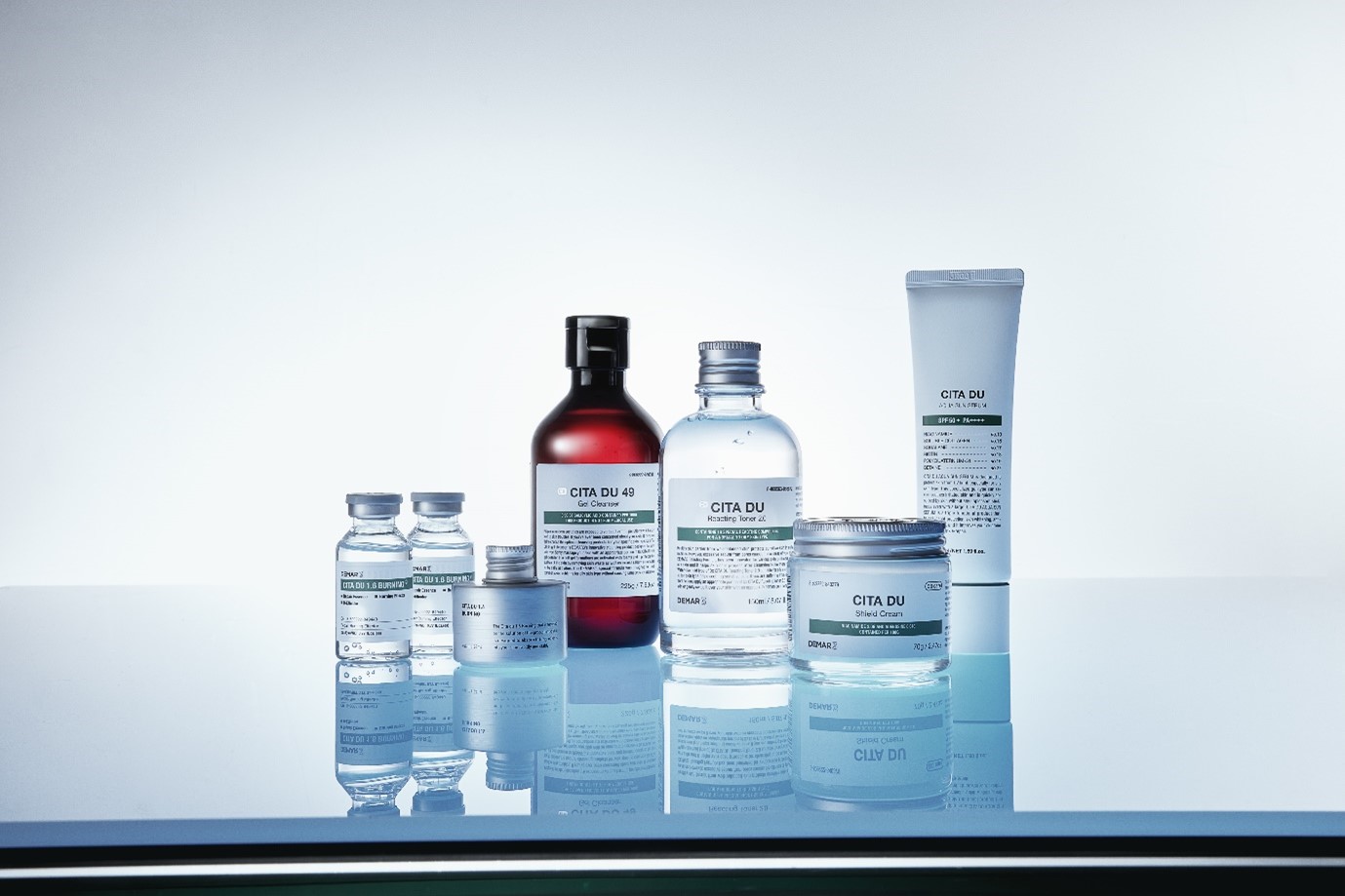
All products of ‘CITA DU’, a large-scale oily skin research project conducted by DEMAR3, were evaluated as hypoallergenic by dermatologists in a human application test conducted at the Skin Clinical Trials Center of OATC. According to the general cosmetics Primary Skin Irritation Index (P.I.I.), 0.00 to 0.25 are considered hypoallergenic products.
Considering that the average P.I.I. of ‘DX CITA DU Burning 1.6 Ampoule 1∙2’, ‘DX CITA DU Reacting Toner’ and ‘CITA DU Shield Cream’ is 0.01, it can be surmised that they cause very little irritation to the skin and is suitable for use on sensitive skin.
Products of CITA DU have been evaluated as being suitable for use on acne-prone skin, which is known as a representative type of skin sensitive to irritation. All facial products, including ‘DX CITA DU Burning 1.6 Ampoule 1∙2’ and ‘CITA DU Shield Cream’ tested in 2022, were judged by dermatologists to be suitable for use on acne-prone skin. Furthermore, since most of the products released through this large-scale project are effective in improving the sebum secretion rate and soothes the skin, it is expected to be of great help to consumers with acne-prone skin.
Currently, ‘DX CITA DU Burning 1.6 Ampoule 1∙2’ and ‘CITA DU Shield Cream’ of DEMAR3 CITA DU are in stock in the US on Amazon. Considering that a wider variety of CITA DU products such as cleansers and sun serums are on sale in the Republic of Korea, consumers around the world are expected to soon be able to find more diverse CITA DU products available.